Lysergic acid, better known by its derivative LSD or lysergic acid diethylamide, has been the focus of much scientific research, cultural movements, and even urban legends. But did you know that it all started with a tiny fungus growing on rye? This fungus, known as ergot, has had an enormous impact, from causing epidemics in the Middle Ages to being the basis for one of the most potent psychedelics we know today.
In this article, we’ll explore together how a tiny spore on a grain of rye can transform into something potent and mysterious. We’ll talk about the history of ergot, how lysergic acid was discovered, and how this discovery changed the course of medicine and popular culture. So sit back, open your mind, and get ready for a mind-blowing journey through the world of fungi, chemistry, and history. Let’s go!
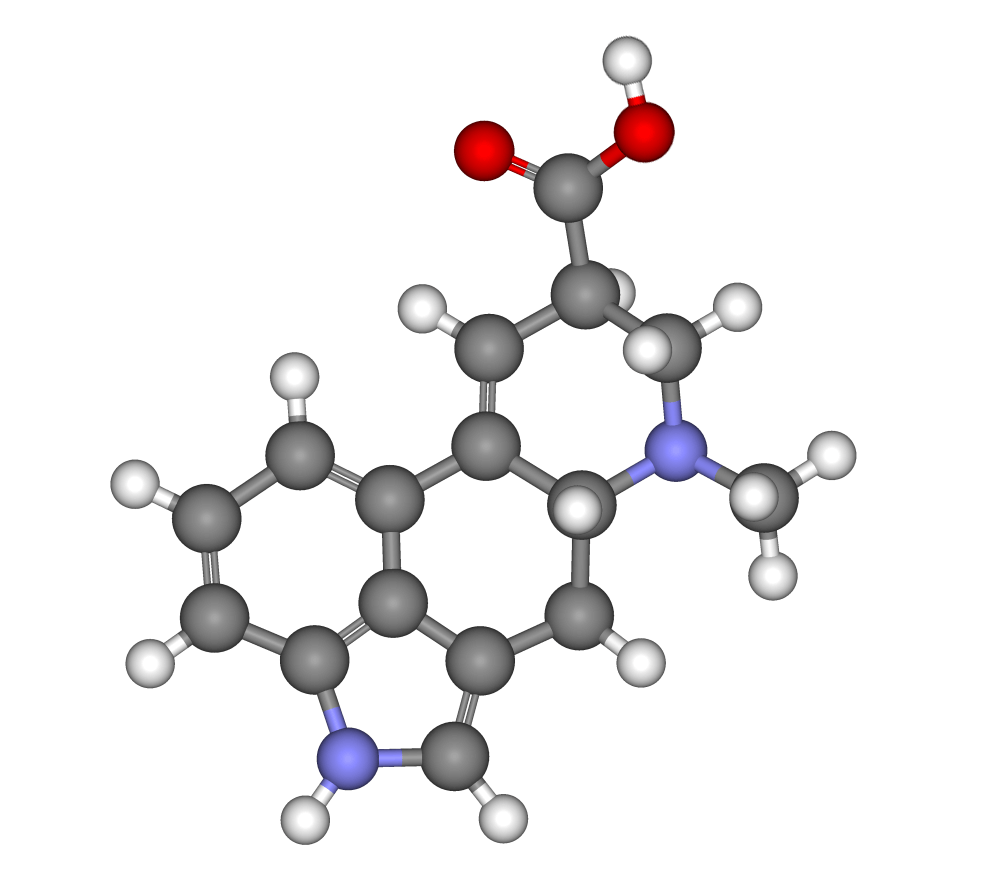
What is lysergic acid?
You’ve probably heard of LSD, right? Well, lysergic acid is a key molecule in the creation of this famous psychedelic, and it has a truly fascinating history and origin. It all starts with a small fungus called ergot (Claviceps purpurea), which grows on rye and other cereal grains. During the Middle Ages, this fungus caused havoc because, when accidentally consumed in bread, it caused serious poisoning and diseases such as ergotism. However, something incredible arose from this problem: scientists discovered that ergot contained very interesting alkaloid chemical compounds, with promising therapeutic properties.
Well, lysergic acid is one of these compounds. In the 1930s and 1940s, a Swiss chemist named Albert Hofmann was researching these compounds and, by chance, managed to synthesize LSD from lysergic acid. This discovery revolutionized both science and culture, as LSD became an important tool in the investigation of the human mind on the one hand and an icon of the counterculture of the 1960s on the other.
But back to lysergic acid itself, as we already know, it is a molecule that forms the basis for creating several compounds, including LSD. In a way, it is a kind of chemical brick that, when modified, can have surprising and truly powerful effects on the human brain, being one of the most well-known and used hallucinogenic substances in the entire world. Today, hallucinogenic mushrooms are usually classified into three large groups: those that produce psilocybin, like most of the magic mushrooms you know, those that produce muscimol, like amanitas, and those that produce lysergic acid, like our protagonist today.

How is lysergic acid produced?
We have seen that lysergic acid is synthesized in the fungus known as ergot, while LSD is a derivative of lysergic acid. This fungus has a rather complex life cycle and during part of that cycle, it produces several alkaloids, including our protagonist today.
Here we summarize how this synthesis process occurs in the fungus:
- Plant infection: Ergot infects cereal plants, primarily rye. When the fungus infects the flowers of the cereal, it replaces the developing grain with a structure called a sclerotium, which is where a large amount of alkaloids are stored.
- Alkaloid production: Within the sclerotium, the fungus produces several chemical compounds, known as ergot alkaloids, which have interesting chemical and pharmacological properties. Lysergic acid is one of these alkaloids, although, in its natural form, it is bound to other molecules to form more complex compounds, such as ergotamine.
- Lysergic acid synthesis: Inside the fungus, lysergic acid is synthesized through a biochemical process involving several steps and specific enzymes. Part of the fungus’ metabolism uses amino acids such as tryptophan, which through a series of enzymatic reactions, leads to the formation of more complex structures, such as lysergic acid. In turn, this acid is a base for the production of other larger and more complex alkaloids.
- Storage in the sclerotium: Once synthesized, lysergic acid and its derivatives are stored in the sclerotium of ergot. These sclerotia are the hard, dark structures that replace infected cereal grains.

In its natural form, lysergic acid is not found free, but rather as part of more complex molecules. To isolate pure lysergic acid, it is necessary to break down these molecules, a process that is carried out in the laboratory. Later, and when chemically manipulated, it can be used to synthesize compounds such as LSD.
Ergotism: effects of lysergic acid
Ergotism, also known as “St. Anthony’s fire” or “St. Anthony’s fever”, is a disease caused by the ingestion of toxic alkaloids produced by fungi such as the one we are concerned with today, ergot. This poisoning can cause two main types of symptoms (convulsive and gangrenous ergotism), which often occur together:
Convulsive Ergotism
This type of ergotism mainly affects the central nervous system and is characterized by:
- Seizures: Violent, uncontrollable muscle spasms that may be painful and prolonged.
- Hallucinations: Disturbing visions and sensations that are often accompanied by paranoia and confusion.
- Headaches: Intense and persistent, often accompanied by dizziness.
- Psychosis: Severe disturbances in the perception of reality, which may include delusions and irrational behavior.

Gangrenous Ergotism
This type of ergotism mainly affects the circulatory system and is characterized by:
- Severe vasoconstriction: Ergot alkaloids cause extreme narrowing of blood vessels, reducing blood flow to the extremities.
- Gangrene: Lack of proper circulation can lead to tissue death, especially in the extremities, causing dry gangrene. This can result in the loss of fingers, hands, feet, and even entire limbs.
- Severe pain: Gangrene is often accompanied by sharp, persistent pain, especially in the affected limbs.
- Skin peeling: In more severe cases, the affected skin may darken and peel off, causing open wounds.
Other effects:
- Nausea and vomiting: Common in the early stages of poisoning.
- Diarrhea: This may occur along with other gastrointestinal symptoms.
- Burning sensation: A classic feature of gangrenous ergotism, giving rise to the term “St. Anthony’s fire” due to the intense burning sensation in the extremities.
Historically, ergotism was responsible for epidemics in the Middle Ages, especially in Europe, when ergot contaminated bread made from rye. This disease caused extreme suffering and, in many cases, death, until its origin was understood and measures were taken to control the fungus in cereal crops. Later, once some of its medicinal properties were discovered, it began to be used as a treatment for various conditions.
From lysergic acid to LSD
The process of converting lysergic acid into LSD (lysergic acid diethylamide) is a chemical procedure that involves a series of carefully controlled reactions. Here’s a quick rundown of how this process works:
- Obtaining lysergic acid: The starting point is lysergic acid, which as we know is obtained from the alkaloids present in Claviceps purpurea. These alkaloids, such as ergotamine, contain the lysergic acid nucleus, which is isolated by chemical processes that eliminate the unwanted parts of the molecule. This isolation is usually carried out in a specialized laboratory due to the complexity and danger of the procedure.
- Activation of lysergic acid: Lysergic acid in its pure form is not yet LSD. To transform lysergic acid into LSD, the molecule must first be activated. This is usually done by reaction with phosgene chloride to form a reactive intermediate called “lysergic acid chloride”.
- Amidation: The key step in converting this intermediate into LSD is an amidation reaction. In this stage, lysergic acid chloride is reacted with diethylamine, an organic compound that introduces the amide group into the molecule. Diethylamine binds to lysergic acid at the specific site, forming the LSD structure.
- Purification: After the reaction, the crude LSD must be purified to remove any byproducts or impurities that may have formed during the process. This is achieved through various methods, such as recrystallization, where the LSD is dissolved in a suitable solvent and then precipitated in a pure, crystalline form.
- Stabilization: Finally, pure LSD is stabilized, as the molecule is sensitive to light and heat, which can quickly degrade it. To preserve its potency, LSD is stored in dark, cool conditions, often in the form of crystals or dissolved in alcohol solutions.
- Formulation: LSD is usually dissolved in a liquid and placed in small doses on blotting paper (known as a ” blotter “), gelatin, or microdots for delivery. These forms allow for precise dosing, as the active amount of LSD is extremely small.
Broadly speaking, the conversion of lysergic acid into LSD is a fairly sophisticated chemical process involving the creation of a reactive intermediate, its reaction with diethylamine, and the subsequent purification of the final product. This procedure not only requires a deep knowledge of organic chemistry but also a controlled environment to ensure the purity and stability of the LSD.
As you can see, the story of ergot and its link to LSD shows us how something as small as a mushroom can have a gigantic impact on science, medicine, and even culture. From the devastating epidemics of ergotism in the Middle Ages to the psychedelic revolution of the 20th century and the use of microdosing in the current century, lysergic acid has taken us on an unexpected journey through the boundaries of the mind and chemistry. This fascinating connection between nature and science remains a reminder that the greatest innovations can emerge from the most unexpected places.
References:
- El cornezuelo del centeno (I): biología, historia y ergotismo, Carlos Illana
- Methods of Lysergic Acid Synthesis—The Key Ergot Alkaloid, Michał Jastrzębski, Agnieszka Kaczor, Tomasz Wróbel
- Overview of the synthetic approaches to lysergic acid as a precursor to the psychedelic LSD, Michael J. Nutt, Nick Woolf, Scott G. Stewart
Source link
#Ergot #lysergic #acid #LSD #Alchimia #Grow #Shop